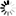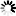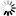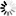Carbapenems are broad-spectrum beta-lactam antibiotics, which are often considered as the antibiotics of last resort. A naturally occurring carbapenem, thienamycin, was first discovered in Streptomyces cattleya. This diagram shows how a simple carbapenem, carbapenem-3-carboxylate, is synthesized from malonyl-CoA and pyrroline-5-carboxylate [MD:
M00675]. For structurally complex carbapenems, such as thienamycin, olivanic acid, epi-thienamycin and carbapenems of the OA-6129 group, uncertainty remains about the mechanism and timing for the inversion of the C-5 carbapenam bridgehead, the desaturation of the C-2/C-3 bond and the attachment of the C-2 and C-6 side chains.
 Carbapenem biosynthesis - Thermus thermophilus JL-18
Carbapenem biosynthesis - Thermus thermophilus JL-18
 Carbapenem biosynthesis - Thermus thermophilus JL-18
Carbapenem biosynthesis - Thermus thermophilus JL-18