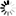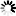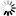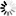N-glycans or asparagine-linked glycans are major constituents of glycoproteins in eukaryotes. N-glycans are covalently attached to asparagine with the consensus sequence of Asn-X-Ser/Thr by an N-glycosidic bond, GlcNAc b1- Asn. Biosynthesis of N-glycans begins on the cytoplasmic face of the ER membrane with the transferase reaction of UDP-GlcNAc and the lipid-like precursor P-Dol (dolichol phosphate) to generate GlcNAc a1- PP-Dol. After sequential addition of monosaccharides by ALG glycosyltransferases [MD:
M00055], the N-glycan precursor is attached by the OST (oligosaccharyltransferase) complex to the polypeptide chain that is being synthesized and translocated through the ER membrane. The protein-bound N-glycan precursor is subsequently trimmed, extended, and modified in the ER and Golgi by a complex series of reactions catalyzed by membrane-bound glycosidases and glycosyltransferases. N-glycans thus synthesized are classified into three types: high-mannose type, complex type, and hybrid type. Defects in N-glycan biosynthesis lead to a variety of human diseases known as congenital disorders of glycosylation [DS:
H00118 H00119].
 N-Glycan biosynthesis - Mustela lutreola (European mink)
N-Glycan biosynthesis - Mustela lutreola (European mink)
 N-Glycan biosynthesis - Mustela lutreola (European mink)
N-Glycan biosynthesis - Mustela lutreola (European mink)